
COVID-19 Vaccine & Heart Conditions
Top 10 COVID-19 Vaccination Tips for People with Heart Conditions
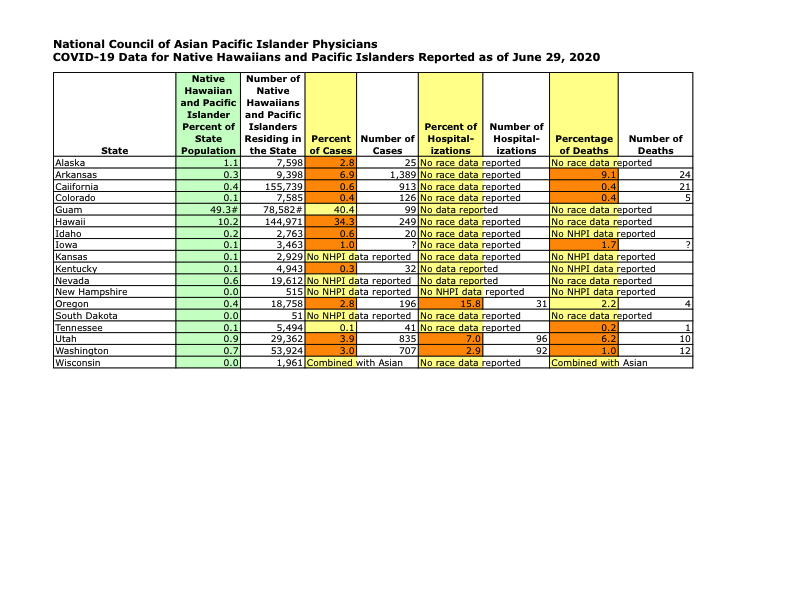
COVID-19 Data for Native Hawaiians and Pacific Islanders Reported as of June 29, 2020
NCAPIP Letter to House Ways and Means Committee Addressing COVID-19 Disparities
The National Council of Asian Pacific Islander Physicians (NCAPIP) submitted this statement to the Ways and Means Committee of the U.S. House of Representatives.
Included in the letter were data that showcased the COVID-19 disparities in Asian Americans and Pacific Islanders, as well as the lack of demographic data collection in many states. The letter also included a multitude of recommendations around culturally competent, linguistically appropriate contact tracing, workforce diversity, and COVID-19 in-language messaging.
The CDC’s interim guidance on COVID-19 contact tracing, issued on May 15, 2020, states:
Culturally and linguistically diverse minority populations are growing in the United States. These populations include racial and ethnic minorities, members of tribal nations, immigrants (i.e., those born outside the United States) and refugees. They may be at higher risk for COVID-19 or worse health outcomes due to a number of reasons including living conditions, work circumstances, underlying health conditions, and limited access to care.
It is important that case investigations and contact tracing are conducted in a culturally appropriate manner, which includes meaningfully engaging community representatives from affected communities, collaborating with community-serving organizations, respecting the cultural practices in the community, and taking into consideration the social, economic and immigration contexts in which these communities live and work.
Currently, CDC contact tracing information is only available in English.
The statement notes that physicians from racial and ethnic and other medically underserved communities can be credible and trusted spokespersons to help educate communities about COVID-19 contact tracing. NCAPIP recommends health departments partner with local medical societies, state medical associations, and racial and ethnic physician organizations to conduct community education about COVID-19 contact tracing.
With the majority of ambulatory care visits being made to small and solo physician practices coupled with their value to vulnerable communities, small practices lack the type of federal support that benefits hospitals, large mainstream practices, and health systems. NCAPIP calls for funding and technical support to be more targeted towards these essential community providers, particularly in response to COVID-19 disparities.
To read the statement: Click Here
Included in the letter were data that showcased the COVID-19 disparities in Asian Americans and Pacific Islanders, as well as the lack of demographic data collection in many states. The letter also included a multitude of recommendations around culturally competent, linguistically appropriate contact tracing, workforce diversity, and COVID-19 in-language messaging.
The CDC’s interim guidance on COVID-19 contact tracing, issued on May 15, 2020, states:
Culturally and linguistically diverse minority populations are growing in the United States. These populations include racial and ethnic minorities, members of tribal nations, immigrants (i.e., those born outside the United States) and refugees. They may be at higher risk for COVID-19 or worse health outcomes due to a number of reasons including living conditions, work circumstances, underlying health conditions, and limited access to care.
It is important that case investigations and contact tracing are conducted in a culturally appropriate manner, which includes meaningfully engaging community representatives from affected communities, collaborating with community-serving organizations, respecting the cultural practices in the community, and taking into consideration the social, economic and immigration contexts in which these communities live and work.
Currently, CDC contact tracing information is only available in English.
The statement notes that physicians from racial and ethnic and other medically underserved communities can be credible and trusted spokespersons to help educate communities about COVID-19 contact tracing. NCAPIP recommends health departments partner with local medical societies, state medical associations, and racial and ethnic physician organizations to conduct community education about COVID-19 contact tracing.
With the majority of ambulatory care visits being made to small and solo physician practices coupled with their value to vulnerable communities, small practices lack the type of federal support that benefits hospitals, large mainstream practices, and health systems. NCAPIP calls for funding and technical support to be more targeted towards these essential community providers, particularly in response to COVID-19 disparities.
To read the statement: Click Here
COVID-19 Treatments Must Work for Communities of Color
Washington, D.C. -- The Alliance of Multicultural Physicians (the Alliance) urged FDA Commissioner Stephen Hahn and members of Congress in a letter to make diversity in clinical trials a greater priority in order to address disparities in COVID-19 cases and deaths in racial/ethnic minority communities, and to address a longstanding lack of diverse participation in clinical trials. The letter came within weeks of an FDA stakeholder call on COVID-19 with the Alliance and other medical groups that advocate for vulnerable populations.
“As a result of the Coronavirus pandemic, a bright light has recently been shown on the health disparities that have always existed in America,” said Dr. Oliver Brooks, President of the National Medical Association (NMA). What the world is witnessing is that Black, Hispanic, Native American, Pacific Islander, and Asian patients are severely overrepresented among those who have suffered the morbidity and mortality of COVID-19.”
Data from the 40 states that collect race and ethnicity data show that White Americans are dying from COVID-19 at a rate of 22.7 per 100,000 in the population, whereas African Americans die at a rate 54.6 deaths per 100,000, Hispanic Americans at a rate of 24.9 deaths per 100,000, and Asian Americans at 24.3 deaths per 100,000. Though the data are sparse for Native Americans, in New Mexico they die from the COVID-19 disease at a rate that is eight times that of the white population, and in Arizona they die at a rate that is five times that of all of others in the population. For Native Hawaiians and Pacific Islanders, the available data in ten states show percentages of COVID-19 cases and deaths that are two to three times greater than their percentage of the population.
The letter, addressed also to the heads of the Pharmaceutical Researchers and Manufacturers of America and the Biotechnology Innovation Organization, states that the majority of approved drug products come with an FDA disclaimer that there is insufficient availability of data to determine effective response in racial-ethnic minority patients due to lack of participants during testing. That problem is often compounded by a high prevalence of the disease in the same minority patients for which the product is indicated for use. "In these difficult times our most marginalized communities remain at the greatest risk for health disparities. COVID-19 has demonstrated what our communities already know, we need to be as One to end health disparities resulting from non inclusion and policies of invisibility in healthcare," said Dr. Brian Thompson, board member of the Association of American Indian Physicians (AAIP). "We appreciate FDA efforts to increase diversity and inclusion in all aspects of medical care."
The Alliance, which includes five national physician associations (AAIP, NMA, NHMA, ABC, and the National Council of Asian Pacific Islander Physicians) acknowledged the FDA and its Office of Minority Health and Health Equity for “its leadership and efforts to encourage diversity in clinical trials, especially with COVID-19 exacerbating and highlighting the health disparities that exists in the United States. Now is the time to support programs to increase clinical trial participation of investigators and participants to reflect the current population of the United States,” said Dr. Elena Rios, President & CEO of the National Hispanic Medical Association. In its letter, the Alliance recommended the FDA provide “clear guidance to all sponsors of medical products directed at COVID-19 to assure inclusion that provides the bases for clinically meaningful data to address the needs of a diverse America.”
Members of the Alliance took note that the path to achieving diverse, reflective clinical trials would take more than regulatory efforts.“An additional focus must be on ensuring that overall or main clinical trial principal investigators are from diverse racial and ethnic groups such as black and Hispanic/LatinX investigators,” Dr. Michelle Albert, President of the Association of Black Cardiologists (ABC). “The latter represents a key upstream component of increasing patient diversity in clinical trials and also improving on current clinical trial strategies and questions. "
The group pledged a continuing effort to address disparities in medicine beyond the duration of the pandemic. “Our country is at a critical juncture on how to move confidently and judiciously to address racial injustice, which permeates every aspect of our social and civic lives,” said Dr. Winston Wong, President and Chair of NCAPIP. “Racial equity must also guide our process of evaluating and approving medical therapy for our communities, especially those that have dealt with generations of inequity.”
“As a result of the Coronavirus pandemic, a bright light has recently been shown on the health disparities that have always existed in America,” said Dr. Oliver Brooks, President of the National Medical Association (NMA). What the world is witnessing is that Black, Hispanic, Native American, Pacific Islander, and Asian patients are severely overrepresented among those who have suffered the morbidity and mortality of COVID-19.”
Data from the 40 states that collect race and ethnicity data show that White Americans are dying from COVID-19 at a rate of 22.7 per 100,000 in the population, whereas African Americans die at a rate 54.6 deaths per 100,000, Hispanic Americans at a rate of 24.9 deaths per 100,000, and Asian Americans at 24.3 deaths per 100,000. Though the data are sparse for Native Americans, in New Mexico they die from the COVID-19 disease at a rate that is eight times that of the white population, and in Arizona they die at a rate that is five times that of all of others in the population. For Native Hawaiians and Pacific Islanders, the available data in ten states show percentages of COVID-19 cases and deaths that are two to three times greater than their percentage of the population.
The letter, addressed also to the heads of the Pharmaceutical Researchers and Manufacturers of America and the Biotechnology Innovation Organization, states that the majority of approved drug products come with an FDA disclaimer that there is insufficient availability of data to determine effective response in racial-ethnic minority patients due to lack of participants during testing. That problem is often compounded by a high prevalence of the disease in the same minority patients for which the product is indicated for use. "In these difficult times our most marginalized communities remain at the greatest risk for health disparities. COVID-19 has demonstrated what our communities already know, we need to be as One to end health disparities resulting from non inclusion and policies of invisibility in healthcare," said Dr. Brian Thompson, board member of the Association of American Indian Physicians (AAIP). "We appreciate FDA efforts to increase diversity and inclusion in all aspects of medical care."
The Alliance, which includes five national physician associations (AAIP, NMA, NHMA, ABC, and the National Council of Asian Pacific Islander Physicians) acknowledged the FDA and its Office of Minority Health and Health Equity for “its leadership and efforts to encourage diversity in clinical trials, especially with COVID-19 exacerbating and highlighting the health disparities that exists in the United States. Now is the time to support programs to increase clinical trial participation of investigators and participants to reflect the current population of the United States,” said Dr. Elena Rios, President & CEO of the National Hispanic Medical Association. In its letter, the Alliance recommended the FDA provide “clear guidance to all sponsors of medical products directed at COVID-19 to assure inclusion that provides the bases for clinically meaningful data to address the needs of a diverse America.”
Members of the Alliance took note that the path to achieving diverse, reflective clinical trials would take more than regulatory efforts.“An additional focus must be on ensuring that overall or main clinical trial principal investigators are from diverse racial and ethnic groups such as black and Hispanic/LatinX investigators,” Dr. Michelle Albert, President of the Association of Black Cardiologists (ABC). “The latter represents a key upstream component of increasing patient diversity in clinical trials and also improving on current clinical trial strategies and questions. "
The group pledged a continuing effort to address disparities in medicine beyond the duration of the pandemic. “Our country is at a critical juncture on how to move confidently and judiciously to address racial injustice, which permeates every aspect of our social and civic lives,” said Dr. Winston Wong, President and Chair of NCAPIP. “Racial equity must also guide our process of evaluating and approving medical therapy for our communities, especially those that have dealt with generations of inequity.”
